D-Day approaches for new medicinal cannabis scheme

With the clock about to expire on a transitional medicinal cannabis scheme, the number of products available to patients is about to be slashed and prices are set to spike. Russell Brown takes a deep dive into the rules that take effect from 1 October 2021, and why it’s so hard to get products approved for sale.
Prescribing medicinal cannabis was so much simpler in the 1950s. Until the World Health Organisation asked our government to ban imports in 1955, bringing us into line with emerging United Nations policy, New Zealanders could go to their doctors and request a tincture of cannabis to treat a migraine or hypertension headache.
In 2021, it is once again possible to be prescribed a cannabis product. But this time around, not much is simple. Nearly 1400 days since then-Opposition leader Jacinda Ardern promised to legalise medical cannabis "within 100 days" of taking office, the birth pains of the regime ushered in by the Misuse of Drugs (Medicinal Cannabis) Amendment Act 2018 continue.
Although the Medicinal Cannabis Scheme has been working fairly well for several thousand patients, it faces a significant challenge in the wake of a decision not to further extend a transitional scheme that has allowed doctors to prescribe products that have not met the "minimum quality standards" set out in The Misuse of Drugs (Medicinal Cannabis) Regulations 2019.
From October 1, all medicinal cannabis products imported into or manufactured in New Zealand must be verified as meeting the minimum quality standards before they can be imported or supplied. The immediate result will be a sharp reduction in the range of CBD-only products available for prescription – and with that, at least a doubling in price. Patients now paying as little as $150 for a CBD product could be facing a near-$400 bill for an equivalent.
All of these products are imported. There are no New Zealand products available because not one product from a cluster of local companies has met the minimum quality standards set out in the regulations since they came into effect on April 1, 2020.
A transitional period of six months was set down to ensure continuity of supply to patients while manufacturers and importers applied to the Medicinal Cannabis Agency, which manages the scheme within the Ministry of Health. During the transitional period, products which met the definition of a CBD product could be freely prescribed by doctors, but any containing THC, a controlled drug, required ministerial approval, which was invariably delegated to ministry officials. It was essentially the situation that had prevailed before the regulations had been published.
Six months later, at the beginning of October last year, no products, local or imported, had met the minimum quality standard. Reasoning that the disruption caused by Covid-19 had hampered companies from providing the necessary information for approval, officials recommended a six-month extension to the transition scheme. As the new March 31 deadline loomed, it was clear only two products – one CBD product and one containing THC, both produced by the Canadian giant Tilray – would meet the minimum quality standards by April 1. The scheme was extended by a further six months, to the end of September.
cannabis plant product next to chemical formula - From October 1, all medicinal cannabis products imported into or manufactured in New Zealand must be verified as meeting the minimum quality standards before they can be imported or supplied.
Meeting the minimum standards
This month, One News reported that one local company, Helius, had met the Good Manufacturing Practice (GMP) benchmark required by the regulations and could be in a position to have a product approved by the end of the year. Within that news story, Little revealed that the transitional period would not be extended beyond its planned expiry at the end of September.
"The feedback I have had from the industry, some of the players," said the minister, "is they don't think it needs to be extended any more, bearing in mind that this is about the safety of products."
From the end of September, New Zealand doctors will have a choice of only four products that have met the minimum quality standards, all from Tilray. Two are CBD-only oils, one is a 50-50 mix of THC and CBD and the other, Tilray 25, is the only THC-only product available in the system. (Doctors can also prescribe Sativex, the THC-CBD tincture approved by Medsafe in 2010, but it's even more expensive and disliked by many patients.)
The contrast between Australia and New Zealand is striking. In Australia, doctors can choose from around 200 cannabis products, including whole cannabis flower – but require individual approval for each prescription. Conversely, New Zealand doctors may prescribe according to their clinical judgement – but have hardly any products at their disposal.
These quality standards are accidentally-on-purpose too high, and they're cancelling better in the pursuit of perfect."
Medleaf principal and former patient advocate Shane Le BrunThe minister's comment that "some of the players" in the fledgling industry did not want the transitional scheme extended again is probably true. Correspondence released to co-founder of the Hemp Foundation Tadhg Stopford under the Official Information Act shows that at least one local company, Helius, opposed the previous extension.
Helius CEO Carmen Doran told Medicinal Cannabis Agency head Andrea Eng in a letter in February that continually moving back the deadline "diminishes the importance of (and trust in) the regulations." Doran was emphatic that GMP accreditation – which Helius has become the first local company to achieve – was essential. But she also said the agency "should exercise discretion and work with medicinal cannabis producers on a case-by-case basis, using a risk-based approach, to allow the supply of products while ensuring appropriate quality."
The principal area where Doran suggested flexibility – product stability – is at the centre of local manufacturers’ struggle to meet regulatory standards. Product stability, or demonstrated shelf life, is a key benchmark for pharmaceutical products. But the requirements of the Medicinal Cannabis Scheme are relatively onerous, requiring six months' stability data for three separate batches of each finished product. It's not uncommon for drug regulators to accept "accelerated data" from products stored in conditions intended to make them degrade more quickly, but New Zealand ministry officials seem to be sticking to the letter of the regulations.
Mark Dye, CEO of NUBU Pharmaceuticals, which imports medicinal cannabis products, also wrote to James in February to say that his company and rival importers Equalis and Medleaf had "scoured the world" in search of CBD products which might meet the minimum quality standards. Dye said the requirement for stability data not only for finished products but individual ingredients in those products had been a particular roadblock – and that Medsafe's inability to audit the GMP status of foreign manufacturers during the pandemic had caused further problems.
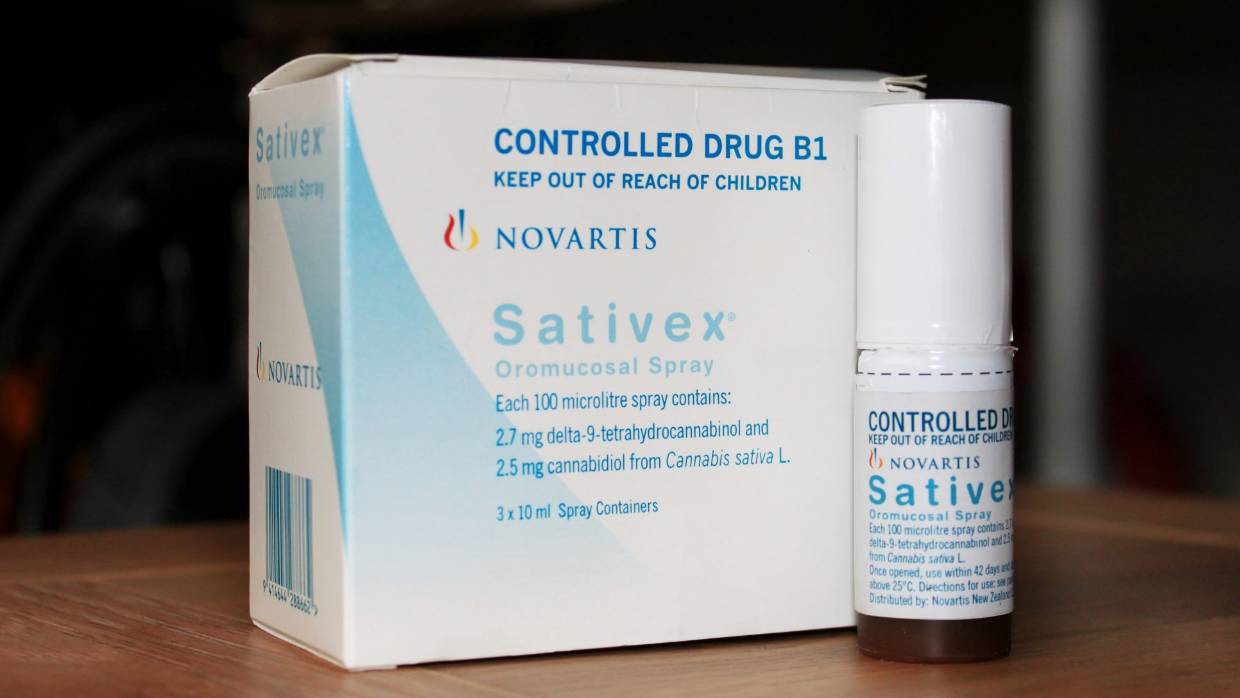
Box of Sativex spray, reads "Controlled Drug B1. Keep out of reach of children... Each 100 microlite spray contains: 2.7mg delta-9-tetrahydrocannabinol and 2.5mg cannabidiol from Cannabis sativa L. 3x 10 ml Spray Containers." - From the end of September, New Zealand doctors will have a choice of only four products. Sativex, approved by Medsafe in 2010, is one of them.
Medleaf principal and former patient advocate Shane Le Brun says his company had offered to fund a remote audit of its Colombian supplier, using a telepresence robot. The supplier, Clever Leaves, already has Croatian GMP certification, chosen as a faster way into the German market. But they had been refused.
"They've refused to spend any bandwidth on even looking at us – and it feels anti-competitive," says Le Brun. "We have the goods, we're offering the cheapest EU GMP product that would meet the standard – for everything apart from one technicality that's just the ministry being poorly-resourced and poorly motivated. These quality standards are accidentally-on-purpose too high, and they're cancelling better in the pursuit of perfect. We could bring in GMP CBD tomorrow, but it might be one or two things deficient compared to our quality standard."
Le Brun says the products Medleaf can't get over the line in New Zealand are acceptable in Germany, Israel and the UK, among others. He says if New Zealand officials would accept EU GMP certification, he would be able to sell CBD for a third of the price per milligram of the Tilray products.
"Talking to international suppliers, the economics of going above and beyond to meet the New Zealand standard is just commercial suicide. Only the very biggest companies will do it. That's why Tilray has got over the line but none of the other Canadian companies have. It just doesn't make sense for them."
An international perspective
Matters of Substance contacted two international producers that Medleaf has worked with, Clever Leaves and the Canadian company MediPharm Labs.
Adam Miller, head of strategic accounts at MediPharm's Australian branch, says New Zealand has some of the strictest cannabis product regulations in the world: “I would go as so far as to say that New Zealand is the most challenging market to register products in. Other highly-regulated markets are more reasonable with their approach.
“[New Zealand's Medicinal Cannabis Agency] has ensured that products meet the highest standards – most companies do not adhere to this level of compliance. This is a double-edged sword. On one hand, the standards are in place to ensure patient safety, but on the other hand, they are so strict that they preclude most companies from entering the market and therefore limit patient options for products that are being prescribed to patients in other counties."
He says other countries – citing Germany and Denmark – require similar standards “but are less stringent on some of the finer details. The MCA would benefit from reviewing the submission requirements from markets like Germany – Germany is highly regulated but also flexible with its approach when good data is presented."
I would go as so far as to say that New Zealand is the most challenging market to register products in. Other highly-regulated markets are more reasonable with their approach."
Adam Miller, head of strategic accounts at MediPharm AustraliaAndrew Miller, Clever Leaves' vice president of sales for Asia-Pacific, says the Colombian company's EU-GMP certification underlines the quality of its THC and CBD products.
"New Zealand has, as all countries do, GMP standards that are in essence, equivalent to those of the EU. It is a matter of finding a way for the New Zealand authorities to officially recognise our EU GMP certification, which we believe is just a matter of time given current COVID-related constraints.
"We believe that providing access for international companies that meet the New Zealand product and manufacturing quality standards would be one way to provide more product offerings, and potentially improved affordability, to New Zealand medical cannabis patients. This could include remote audits being performed for those companies that are already EU-GMP certified."
So why is it so hard?
Manu Caddie, co-founder of the NZSX-listed Rua Bioscience and chair of industry body the Medical Cannabis Council, says producing GMP-standard medicines is “a complex process that includes a massive amount of documentation and tightly-controlled management. Doing this for drugs made synthetically in a lab is difficult, doing it from a fickle plant is even more difficult. It is not impossible but it takes a lot of time to prove to auditors that the process is absolutely robust and any risk of contaminated or inconsistent medicines reaching sick patients is eliminated."
Rua, like all the aspiring local producers, has been diplomatic in public, but is now circulating a graphic which summarises and compares New Zealand's cannabis medicine regime with those of Australia and Germany. In short, it finds that New Zealand's product regulations, especially around product stability, are much more stringent than those of either country, with little or no flexibility in their application.
Another regulatory decision causing some consternation in the industry is the permitted microbial count. The European Pharmacopoeia, on which the New Zealand regulations draw heavily, offers two standards for inhaled products and our officials chose the lower (and hence more stringent) one, which was designed for medicines like powder inhalers for asthma. One person in the industry told Matters of Substance that testing had shown the stricter standard was unworkable: "You fail the test when you open the bag."
Rua's chief operations officer Paul Naske says microbial counts are important in medicines that could be inhaled by cancer patients, but the adoption of the stricter standard has implications for dried flower products – which the New Zealand scheme theoretically permits.
"If you're going to grow this product outside, I mean, everything's got microbes on it. There's lots of yeast moulds in the air and the atmosphere, kiwifruit and apples have all got it," says Naske. "And that sort of drives you down the path of indoor growing."
The alternative – gamma irradiation – would help cannabis flower meet the standard, but may not be palatable for some customers.
But there's more
There are other wrinkles. Section 2A of Misuse of Drugs Act regulations says that "specified substances" in a CBD product can be equivalent to no more than 2% of the total quantity of CBD – even slightly over, and it's no longer a CBD product but a controlled drug. The specified substances are THC, THCA (which is not psychoactive) and their isomers.
That's the problem faced by Auckland mother Katy Thomas, who has been importing CBD oils from the UK company CBD Brothers, under prescription from a GP, to treat her son Eddy's tonic-clonic seizures, which can be life-threatening. According to the World Health Organisation, CBD enjoys more evidential backing as a treatment for childhood epilepsy than it does for any other condition.
Thomas has seen two shipments of the oil seized at the border because a specified substance was slightly over the two-per-cent-of-CBD limit. She says the cannabis indica-based CBD Brothers oil is the only one that works well for Eddy.
Ironically, the oil in question doesn't contain any THC. But 0.136% by volume of the last seized batch was CBN, a minor cannabinoid not specifically mentioned in the regulations, that may have a mild psychoactive effect. Had the oil contained a slightly higher dose of CBD, the relative proportion of CBN would have been lower and it wouldn't have been seized.
"It's negligible," says Thomas, "and I just can't see how enforcing this regulation on a very sick, very dependent child outweighs the benefit of his life."
Thomas eventually took a punt on buying an entire batch – enough till the end of the year – with a certificate of analysis that said it was compliant.
Even with Medsafe approval she was worried about clearing Customs, but the delivery got through.
In the course of it all, Thomas changed doctors – Eddy's former GP couldn't cope with the weight of compliance and was wary of being found to have attempted to import a controlled drug. Waseem Alzaher, CEO of Auckland's Cannabis Clinic, has taken over prescribing for Eddy.
"I'm not aware of any other country that defines a CBD product the way we do," says Alzaher. "It doesn't make any sense."
In fact, the 2% threshold was adopted from Australia in 2016, when the Expert Advisory Committee on Drugs was considering its advice on re-scheduling CBD, and acknowledged that a 100% pure CBD product was impractical. The result was Section 2A, which defines a "specified substance" for the purposes of the threshold. The Thomas family's ordeal seems to rest on the official interpretation of that definition, which, in the ministry's view, captures CBN. Whether the presence of CBN is harmful (or even beneficial) is not an issue.
Thomas believes the entire scope of the law needs changing, "because it's not meeting anybody's needs – patients or prescribers" and that CBD should be removed from the Medicines Act to make it more accessible.
Although it has been deemed safe by the WHO, at higher doses CBD can interact with other medicines – much like grapefruit does. If the intention of keeping it as a prescription medicine was that prescribers would manage such risks, we're not there yet.
"Nobody knows anything!" says Thomas. "Prescribers really rely on your knowledge, because there's been nothing handed down from the government. The prescriber information that's currently being developed by bpac should have been online years ago. That would have given prescribers the literature that they need to dose and titrate and help patients when they ask for it. But they don't have that."
Even when more local producers are able to pass the GMP hurdle and begin to get products over the line, it's unlikely that prices will fall, especially for CBD, which is dosed in higher quantities than THC. Caddie says New Zealand could follow the US and allow CBD to be derived from industrial hemp, and adopt Australia's move to allow low-dose CBD to be sold over the counter.
But any significant change to the regime seems unlikely in the short term. In written answers to Matters of Substance, Medsafe group manager Chris James said there are “no intentions to implement broad reform of the Medicinal Cannabis Scheme" and that it would take time for the benefits of the scheme to be realised. He expected the arrival of local products to “affect costs" but warned that would take "some time" to happen. A ministry spokesperson confirmed after the decision not to further extend the transitional scheme that "no changes to the broader medicinal cannabis scheme are being considered at this stage."
Who can qualify for an exemption?
One part of the 2018 law is, however, being looked at: A review of the palliative exemption which provides a statutory defense for patients using illegal cannabis products is underway. Any expansion of the exemption beyond those with terminal conditions, to include patients with chronic illness, has already been ruled out.
But it might not be quite that straightforward. When the exemption was being written into law, it was originally proposed that a patient would need to be six months from dying to qualify. After select committee submissions, it was decided that 12 months would be more compassionate. But the clock ran out on alterations to the bill before a timeframe could be added.
Auckland patient advocate Pearl Schomburg was interviewed for the review and says she was told by her interviewer that any condition requiring palliative care would qualify.
"She said, 'your rheumatoid arthritis, which can never be cured, it's only ever going to be managed, that would be one of the many conditions that which would come under that palliation exemption'," says Schomburg.
The absence of a time-limit is also reflected in a letter template distributed by Dr Graham Gulbransen and others, which allows a doctor to declare that a patient "has an advanced progressive life-limiting condition and is nearing the end of their life", without citing a time-frame. Dr Gulbransen told Matters of Substance that he could not recall the exact source of the template, but was sure it had come from a ministry website.
Ironically, amid a story of regulations seen as unnecessarily strict, this appears to be a case of the law being accidentally kind.
What happens come October
The review will report to Health Minister, Andrew Little, in December. But for now, the looming challenge for producers, importers and patients is the end of the transitional regime after September 30.
Official Information Act requests for advice and correspondence around the decision not to extend the transitional scheme again will take some weeks to bear fruit, but earlier advice shows what officials regarded as the risks of not continuing the scheme.
"Feedback from the health sector indicates that disruption to the availability and cost of products would have a significantly negative effect on patient wellbeing," the ministry's report warned Minister Hipkins in September last year. "If the transitional period is not extended, it will reduce access to products for patients who are currently accessing them to appropriately manage their health conditions."
A subsequent report to the new Health Minister, Andrew Little, in February warned of the "ongoing risk" that "Patients who might not be able to afford approved products … could turn to the illicit market."
It's doubtful the situation has changed enough in the past six months that the warnings to two successive health ministers no longer apply.
Recent news

Reflections from the 2024 UN Commission on Narcotic Drugs
Executive Director Sarah Helm reflects on this year's global drug conference
What can we learn from Australia’s free naloxone scheme?
As harm reduction advocates in Aotearoa push for better naloxone access, we look for lessons across the ditch.
A new approach to reporting on drug data
We've launched a new tool to help you find the latest drug data and changed how we report throughout the year.

